Research Areas
The long-term research goals of the So Lab are three-fold: (i) to understand charge and energy transport pathways and behavior, (ii) to elucidate mechanisms and kinetics at interfaces of inorganic-organic interfaces, and (iii) to discover new metal-organic coordinated assemblies. We will probe morphological, structural, optical, photophysical, electrochemical, and optoelectronic property to better elucidate the physico-chemical behavior of self-assembled nanomaterials. The resulting work will add insight to the criteria needed to rationally design multi-functional nanomaterials for energy conversion, energy storage, water purification, food production, and catalysis.
The long-term research goals of the So Lab are three-fold: (i) to understand charge and energy transport pathways and behavior, (ii) to elucidate mechanisms and kinetics at interfaces of inorganic-organic interfaces, and (iii) to discover new metal-organic coordinated assemblies. We will probe morphological, structural, optical, photophysical, electrochemical, and optoelectronic property to better elucidate the physico-chemical behavior of self-assembled nanomaterials. The resulting work will add insight to the criteria needed to rationally design multi-functional nanomaterials for energy conversion, energy storage, water purification, food production, and catalysis.
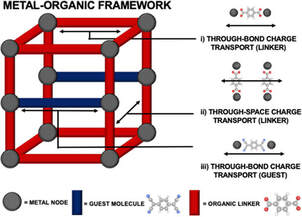
Charge Transport in Metal-Organic Frameworks (MOFs)
As you can see from the name, MOFs are built from two different components - the first being metals and the second are the organic building blocks. You can mix and match metal and organic building blocks, like a kid at a LEGO store. We like to compare MOFs to programmable nanosponges - programmable because instead of focusing on single elements on the periodic table (like carbon), the entire periodic table is open for making and modifying MOFs. MOFs are like nanosponges because they have so many pores. In fact, one tablespoon of MOF powders is so porous that if you lay them out flat, they have the internal surface area of a standard sized football field!
One potential application for conductive high surface area materials is lithium ion batteries that you’re using in your laptops or cell phones everyday. If we can make a solid electrolyte that lets lithium ions walk through fast, you get a renewable battery with high power density. We use a three stage process in MOF research - synthesis, characterization, and evaluation. In the first stage, MOFs are synthesized from a process called self-assembly - that means mixing the metals and organic building blocks in one pot and then programming them such that the building blocks find each other in a specific fashion to get one product in almost 100% yield. In the second stage, we can use powder x-ray diffraction technique to solve the structure, followed by infrared and Raman spectroscopies to determine the organic groups in the MOFs, and finally elemental analysis to confirm the metal ions (or clusters) in the MOFs. In the third stage, we evaluate the MOFs, depending on which application we’re targeting. For instance, we can transform these loose MOF powders into pellets by applying over 2 tons of pressure so we can do conductivity measurements on the MOFs to evaluate their conductivity. See representative work here and here. This work is generously supported by the National Science Foundation and Department of Energy.
As you can see from the name, MOFs are built from two different components - the first being metals and the second are the organic building blocks. You can mix and match metal and organic building blocks, like a kid at a LEGO store. We like to compare MOFs to programmable nanosponges - programmable because instead of focusing on single elements on the periodic table (like carbon), the entire periodic table is open for making and modifying MOFs. MOFs are like nanosponges because they have so many pores. In fact, one tablespoon of MOF powders is so porous that if you lay them out flat, they have the internal surface area of a standard sized football field!
One potential application for conductive high surface area materials is lithium ion batteries that you’re using in your laptops or cell phones everyday. If we can make a solid electrolyte that lets lithium ions walk through fast, you get a renewable battery with high power density. We use a three stage process in MOF research - synthesis, characterization, and evaluation. In the first stage, MOFs are synthesized from a process called self-assembly - that means mixing the metals and organic building blocks in one pot and then programming them such that the building blocks find each other in a specific fashion to get one product in almost 100% yield. In the second stage, we can use powder x-ray diffraction technique to solve the structure, followed by infrared and Raman spectroscopies to determine the organic groups in the MOFs, and finally elemental analysis to confirm the metal ions (or clusters) in the MOFs. In the third stage, we evaluate the MOFs, depending on which application we’re targeting. For instance, we can transform these loose MOF powders into pellets by applying over 2 tons of pressure so we can do conductivity measurements on the MOFs to evaluate their conductivity. See representative work here and here. This work is generously supported by the National Science Foundation and Department of Energy.
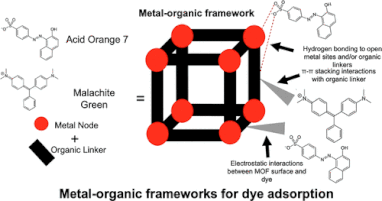
Interfacial Phenomena Between Nanostructured Materials and (A)Biotic Surfaces
Another application of high surface area MOFs is water filter enhancers; since MOFs have tiny pores that block out many large impurities, they can soak up toxic heavy metals (like mercury and lead) or carcinogenic compounds, which leads to birth defects, organic damage, and cancer. The MOF filters can be reused several times. If scaled up, the new material can provide much-needed help to nearly 1 billion people who are unable to access clean drinking water globally. We examine the chemical and physical conditions that impact the adsorption and absorption of charged ions and dyes on MOF-decorated surfaces of (a)biotic substrates, and then use UV-vis or atomic absorption spectroscopies to kinetically determine how much charged species is being removed by the MOF-decorated surface. See representative work here and here. This work is generously supported by the Lantis University Foundation.
Another application of high surface area MOFs is water filter enhancers; since MOFs have tiny pores that block out many large impurities, they can soak up toxic heavy metals (like mercury and lead) or carcinogenic compounds, which leads to birth defects, organic damage, and cancer. The MOF filters can be reused several times. If scaled up, the new material can provide much-needed help to nearly 1 billion people who are unable to access clean drinking water globally. We examine the chemical and physical conditions that impact the adsorption and absorption of charged ions and dyes on MOF-decorated surfaces of (a)biotic substrates, and then use UV-vis or atomic absorption spectroscopies to kinetically determine how much charged species is being removed by the MOF-decorated surface. See representative work here and here. This work is generously supported by the Lantis University Foundation.
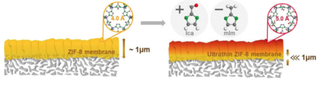 Figure adapted from Lee et al. Angew. Chem. Int. Ed., 2018, 57(1), 156-161.
Figure adapted from Lee et al. Angew. Chem. Int. Ed., 2018, 57(1), 156-161.
Separation of Petroleum-Relevant Products in Selective Nanostructured Membranes
Unlike traditional distillation methods which separate by only discrete boiling points, membrane-based separation technologies fractionate based on molecular differences in size, shape, and membrane-penetrant interactions. However, membrane-based technologies have limitations, such as an intrinsic tradeoff between permeability and selectivity, as well as limited chemical, synthetic, and pore size tunability. Therefore, we propose tunable and selective MOF-based membranes that address these shortcomings. The objective of this work is to investigate the tunability of MOF membranes for selective separation of light olefins (ethylene, propylene) from paraffins (ethane, propane) and map the structure-property relationships governing membrane-based separations processes of petroleum. See representative work here, here, and here. This work is generously supported by the American Chemical Society's Petroleum Research Fund.
Unlike traditional distillation methods which separate by only discrete boiling points, membrane-based separation technologies fractionate based on molecular differences in size, shape, and membrane-penetrant interactions. However, membrane-based technologies have limitations, such as an intrinsic tradeoff between permeability and selectivity, as well as limited chemical, synthetic, and pore size tunability. Therefore, we propose tunable and selective MOF-based membranes that address these shortcomings. The objective of this work is to investigate the tunability of MOF membranes for selective separation of light olefins (ethylene, propylene) from paraffins (ethane, propane) and map the structure-property relationships governing membrane-based separations processes of petroleum. See representative work here, here, and here. This work is generously supported by the American Chemical Society's Petroleum Research Fund.
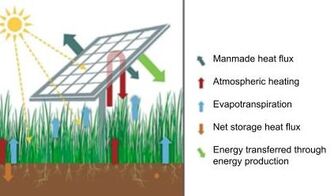
Light Intensity Effects of Semi-transparent Agrivoltaics on Plant Growth
In California, projected heat and drought caused by climate change threatens water, food, and energy security. Existing water scarcity in conventional agricultural settings may reduce food production by up to 40% in arid regions. In fact, as a result of overreliance on irrigation to grow non-dryland-adapted food within arid climates of southern California, water scarcity has led to the conversion of croplands to renewable energy developments at a rate of 20,000 acres within a single year! While this change in land use from agriculture to energy production helps meet the projected 150% growth in global energy demands by 2050, it results in land taken out of food production. Our research focuses on finding solutions for balancing the demands on water, food, and energy resource systems in California using an integrative approach like agrivoltaics, or the colocation of agriculture and solar photovoltaic (PV) infrastructures. Agrivoltaics enable simultaneous crop growth and electricity production on the same agricultural land, potentially increasing land productivity by up to 70% and the value of energy production systems by 30%. In addition to these benefits, agrivoltaics has the potential to reduce plant stress, increase soil moisture, and optimize PV panel performance. We evaluate the effects of light intensity from semi-transparent organic photovoltaic-based agrivoltaics on plant's photosynthetic activity, growth parameters, and yield. See representative work here, here, here, and here. This work is done in collaboration with Dr. Kathleen Meehan at CSU Chico's Department of Electrical and Computer Engineering and generously supported by the Agricultural Research Institute.
In California, projected heat and drought caused by climate change threatens water, food, and energy security. Existing water scarcity in conventional agricultural settings may reduce food production by up to 40% in arid regions. In fact, as a result of overreliance on irrigation to grow non-dryland-adapted food within arid climates of southern California, water scarcity has led to the conversion of croplands to renewable energy developments at a rate of 20,000 acres within a single year! While this change in land use from agriculture to energy production helps meet the projected 150% growth in global energy demands by 2050, it results in land taken out of food production. Our research focuses on finding solutions for balancing the demands on water, food, and energy resource systems in California using an integrative approach like agrivoltaics, or the colocation of agriculture and solar photovoltaic (PV) infrastructures. Agrivoltaics enable simultaneous crop growth and electricity production on the same agricultural land, potentially increasing land productivity by up to 70% and the value of energy production systems by 30%. In addition to these benefits, agrivoltaics has the potential to reduce plant stress, increase soil moisture, and optimize PV panel performance. We evaluate the effects of light intensity from semi-transparent organic photovoltaic-based agrivoltaics on plant's photosynthetic activity, growth parameters, and yield. See representative work here, here, here, and here. This work is done in collaboration with Dr. Kathleen Meehan at CSU Chico's Department of Electrical and Computer Engineering and generously supported by the Agricultural Research Institute.
